The extinction of the Passenger Pigeon was a sobering lesson for Americans. A successful effort to bring it back would demonstrate the potential of genomic intervention and help to restore the ecology of North America’s eastern forests.
PASSENGER PIGEON PROJECT
ABOUT
The Great Passenger Pigeon Comeback began in 2012 with a central paradigm: de-extinction needed a model candidate. The goal of de-extinction for us, quite literally is revive and restore, and so the pilot project needed to be one that would have a chance of successfully returning the species to the wild.
We hypothesized the Passenger Pigeon could be a model de-extinction project. The Passenger Pigeon is certainly an iconic candidate. Conservation has often rallied behind iconic birds to galvanize environmental revolutions. The conservation movement itself formed in response to the extinction of the Passenger Pigeon. When the last birds were shot in the wild, mere decades after their population numbered in the billions, their absence from the skies demonstrated that even the most abundant of natural resources could be exhausted by unchecked human consumption, beginning a new age of conservation regulation and game management.
The Passenger Pigeon and the Future of Forest Conservation
Our research revealed the Passenger Pigeon isn’t simply a model species; it quite possibly is the most important species for the future of conserving the woodland biodiversity of the eastern United States. As a result, the project is now not only a model for pioneering de-extinction methods, but it offers a new opportunity to achieve long-term conservation goals for woodland forests in the eastern U.S.
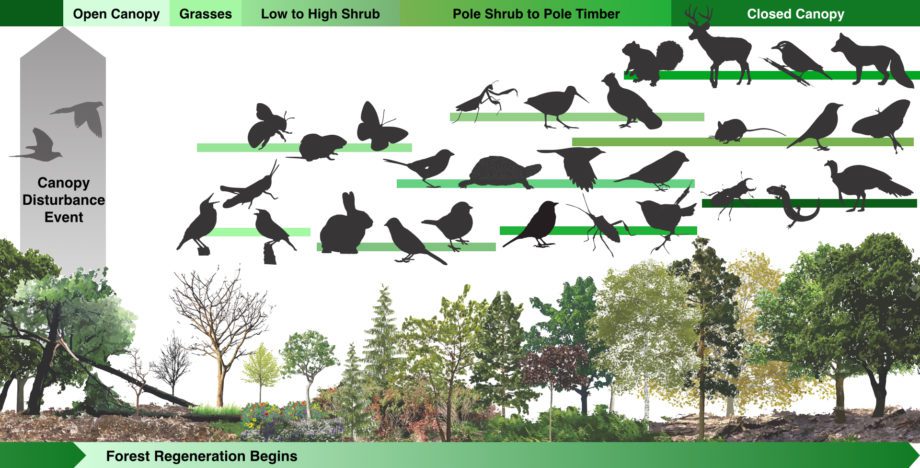
Like many forest ecosystems, North America’s Eastern forests rely on disturbance as an integral factor in regenerating forest cycles. Regular, periodic, mild to moderate disturbances are a positive element in forest ecology. Widespread, asynchronized disturbances produce a mosaic patchwork landscape of different regenerative stages – known as successional stages. Forestry ecologists now recognize that patchwork mosaic forests support greater diversity of species than even-aged stands of trees. Regenerating forest habitats are also more bioproductive, generating higher rates of photosynthesis and carbon sequestration.
This graphic depicts changes in the ecological community following a canopy disturbance – such as those historically created by passenger pigeon flocks. After a canopy disturbance an open canopy habitat allows sunlight to stimulate the successive colonization of grasses, flowers, shrubs, and thicket. Communities of insects, amphibians, reptiles, birds, and mammals all change as old trees regenerate, new trees germinate and mature, and the forest matures to a closed canopy once again.
The majority of native animal species of eastern North America use regenerating forest habitats to feed and raise young. Many plants, including oak trees and the American Chestnut tree, require disturbances and early successional stages to complete their life cycles. A number of early successional bird species have been the focus of conservation concern for ornithologists as the eastern forests have matured into later stages. Many of these species are currently declining, threatened, or endangered in the absence of regular disturbance and subsequent regenerating habitats.
Through Revive & Restore’s de-extinction research, we discovered that large dense flocks of Passenger Pigeon were a significant driver of forest disturbances for tens of thousands of years. While the eastern U.S. has experienced a remarkable reforestation over the past seventy-five years, significant disturbance regimes have been reduced to weather events and logging activities, reducing the healthy mosaic of successional stages and leaving populations of many native inhabiting species in decline. Also, wildfire suppression efforts removed another consistent source of positive forest disturbance. By restoring abundant and densely social flocks of Passenger Pigeons to the eastern U.S., we can restore a consistent and significant disturbance factor to stimulate cycles of regeneration, making forests more productive and diverse.
HOW TO MAKE A PASSENGER PIGEON
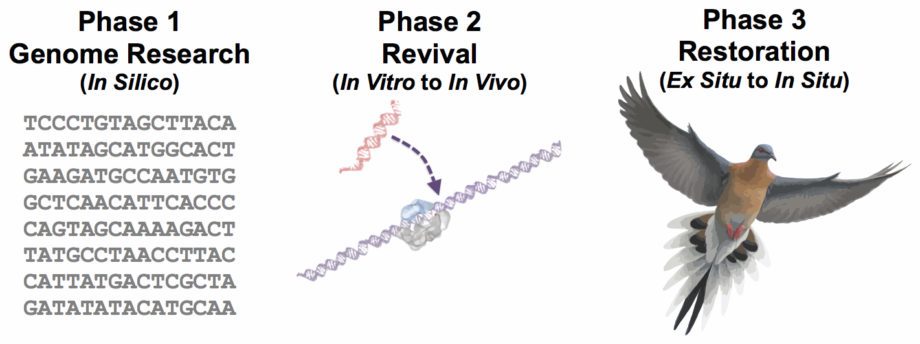
Developing de-extinction science for the Passenger Pigeon has helped define the scientific path for the genetic rescue of all bird species. This path consists of five stages from research to restoration: in silico, in vitro, in vivo, ex situ, and in situ. These five stages can be grouped into three overarching phases given their interdependent technologies and overlapping resource requirements: genome research, revival, and restoration.
For the Passenger Pigeon, the five stages of de-extinction are:
- Comparing the genomes of the Passenger Pigeon and Band-tailed Pigeon
- Identifying regions of the living Band-tailed Pigeons genome to edit
- Editing the germ-line of living Band-tailed Pigeons
- Breeding a new generation of Passenger Pigeons in captivity
- Reintroducing Passenger Pigeons to the wild through proper conditioning and monitoring.
For a detailed breakdown of how we envision Passenger Pigeon de-extinction click through the tabs below.
De-Extinction Project Phases
In Silico Genome Research: The Band-tailed Pigeon Genome
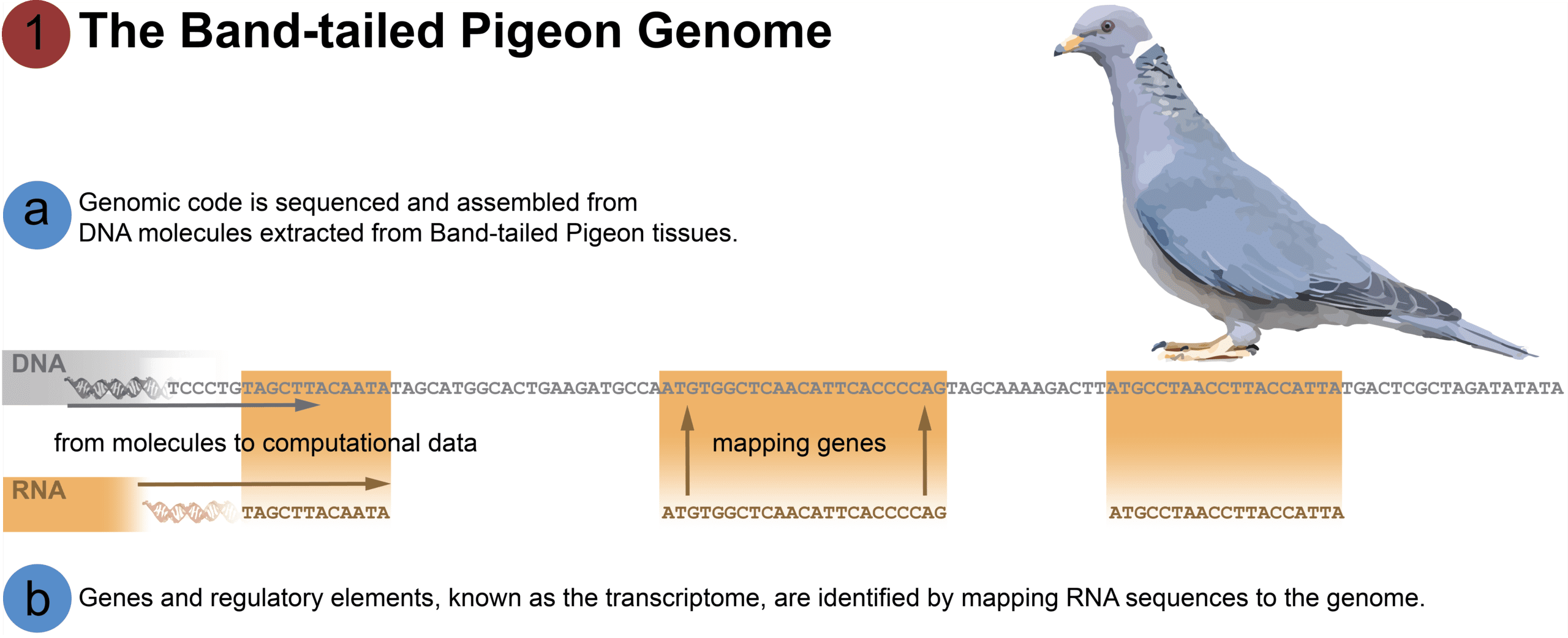
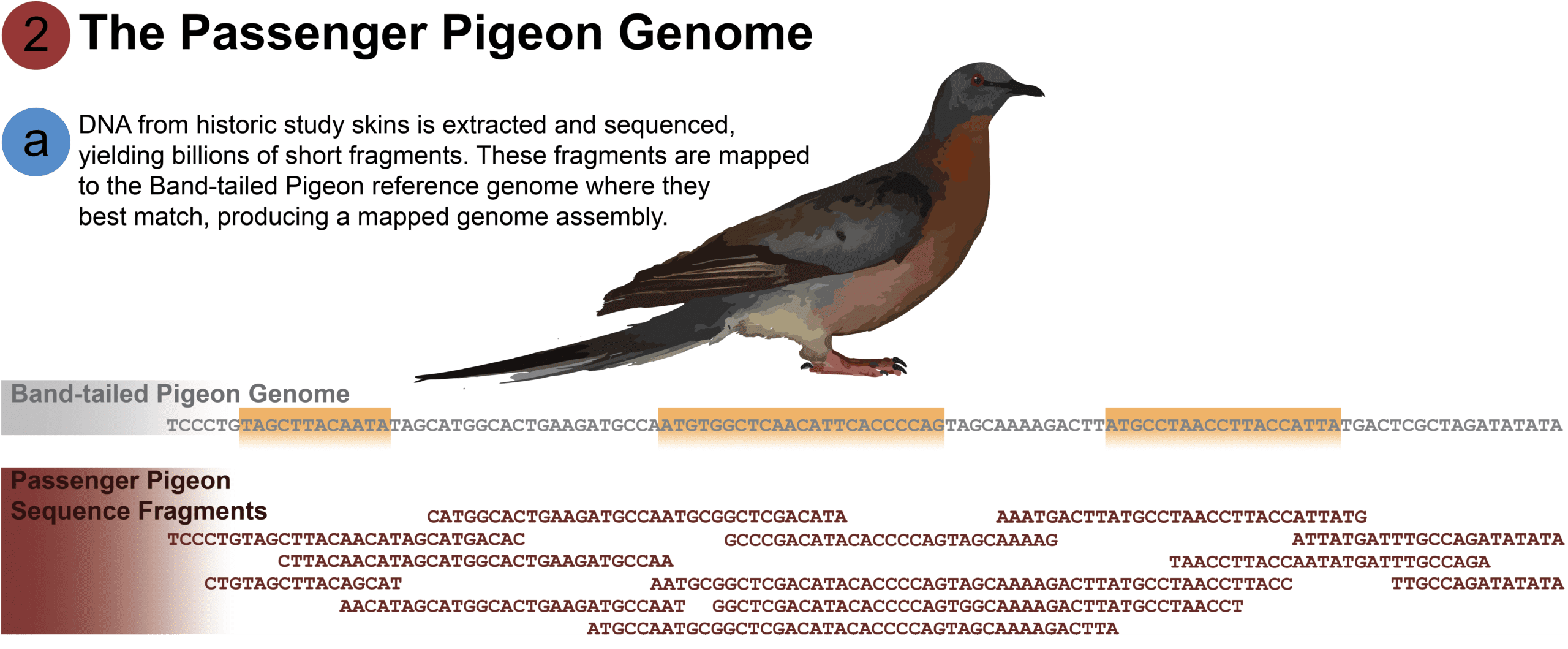
[A] DNA from fresh Band-tailed Pigeon tissue was obtained from a blood sample from “Sally,” a female Band-tailed Pigeon raised by professional breeder Sal Alvarez. The DNA code was sequenced, and the resulting data assembled computationally to produce the genome of the species. The end product is approximately 1.1 billion base pairs of genetic code. However, this genome is simply a string of A’s, T’s, C’s, and G’s. The individual genes and gene regulators are not known; these must be identified by sequencing the Band-tailed Pigeon’s RNA and mapping them to the genome.
[B] In order to determine which sections of the genetic code are used to regulate cell functions and to create traits, the RNA needs to be sequenced. DNA controls traits by being transcribed (written) into RNA, which is then used by the cell to synthesize proteins and regulate how little or how much protein is made. All these elements in the genome that are processed through an intermediate RNA stage are known as the transcriptome. Birds’ genomes contain ~20,000 encoded elements interspersed on different chromosomes. Different tissues in the body express different subsets of these RNA elements – therefore to discover and map them all we need to sequence RNA from multiple tissues. We sequenced RNA from the brain, heart, lungs, liver, kidney, and ovary tissues of one of Sally’s embryonic offspring to identify the protein coding genes of the genome.
Our reference genome is publicly available on the National Center for Biotechnology Information’s public GenBank database. Future work will continue to identify the non-coding elements of the transcriptome.
In Silico Genome Research: The Passenger Pigeon Genome
It is not possible to assemble the genome of the Passenger Pigeon in the same way we can assemble the Band-tailed Pigeon genome – the ancient DNA of the Passenger Pigeon is too fragmented to obtain a contiguous code. Instead, we use the Band-tailed Pigeon reference genome as a template to map the Passenger Pigeon’s fragmented DNA. There is one more problem that complicates the mapping process; ancient DNA is riddled with degraded code.
However, we can “read through” those false mutations in the code by sequencing enough fragments to overlap and form a consensus. The frequency with which DNA fragments overlap at every base pair of the reference genome is what genomicists refer to as depth coverage. In the above image, the Passenger Pigeon genome ranges from 1X coverage on the far left (a single read mapped to the reference base pair) to 5X in the middle (5 overlapping reads per reference base pair). Our partners at the UCSC Paleogenomics Lab sequenced the genomes of two Passenger Pigeons to a high standard of depth coverage of 50X each, enabling researchers to accurately piece together the genomes.
Phase 1.3 – In Silico Genome Research: Genome Comparisons
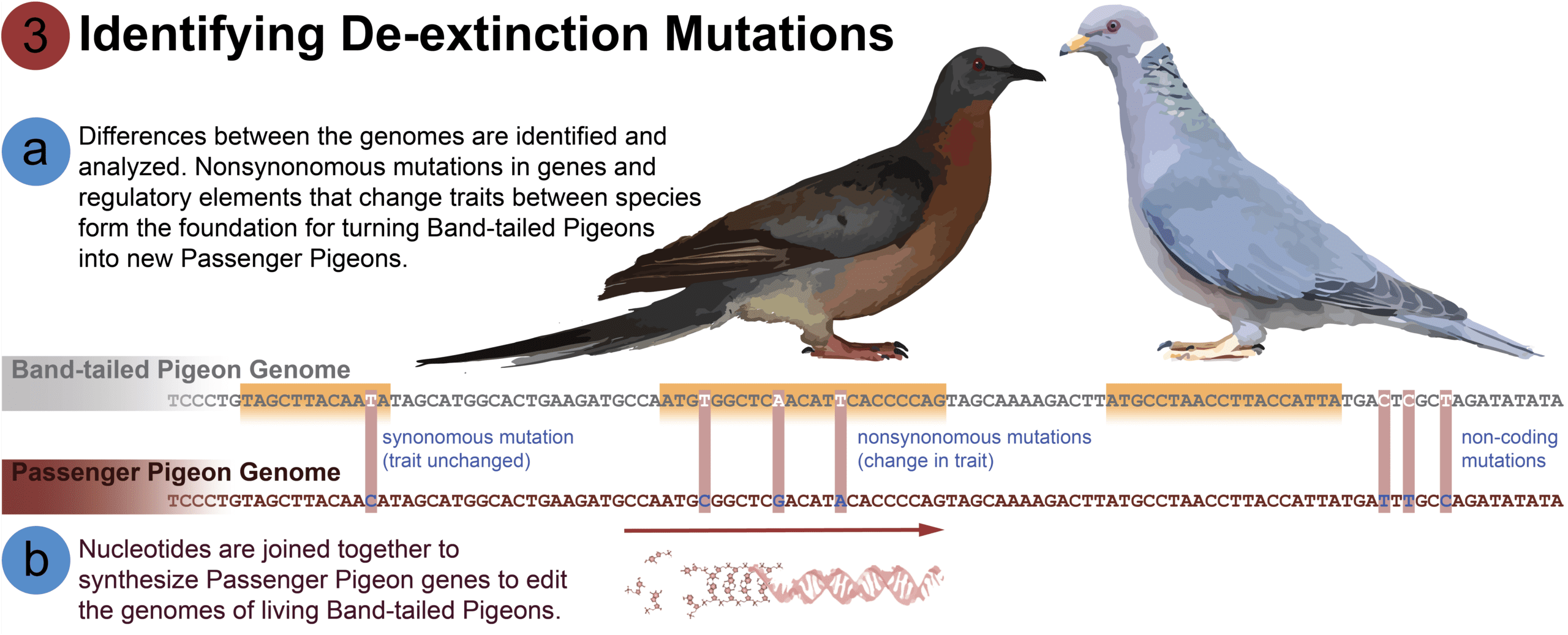
[A] Some mutations matter to de-extinction while others do not. Consider this example: your parents and siblings all have unique mutations, but you are all human. Those unique mutations make you different from one another, but are not what make you different than a chimpanzee. The mutations that make you different from a chimpanzee are shared by all humans. Therefore, unique diversity is not evolutionarily significant for discovering the shared traits that make a Passenger Pigeon a species, though they will be important later for developing genetic diversity in a viable population. These shared mutations are called fixed differences.
By scanning the genomes of the Passenger Pigeon and Band-tailed Pigeon side by side, we can identify the fixed differences. Not all of these differences affect traits. Some mutations are silent, meaning they are not coded into proteins. These silent mutations build up over time by a process called genetic drift and are not acted on by evolutionary selection. The fixed mutations that change traits will form the blueprint of the Passenger Pigeon. Of the ~168,000 fixed differences we have identified in protein-coding genes, 37% of those mutations actually make real changes, affecting 66% of the total number of protein-coding genes, distinguishing the Passenger Pigeon’s phenotypes from those of the Band-tailed Pigeon. Though, despite differences in their amino acid chains, it’s likely that the majority of the two species’ proteins function in the same way. The next step in research is to decipher which proteins have altered functions and which of those might influence the ecology of the two species.
[B] Once the fixed differences are identified, we will make the genetic code into viable DNA molecules, which can then be used to edit the living genome of Band-tailed Pigeons. Our end goal is a library of digital and synthesized Passenger Pigeon DNA that will serve as the building blocks for de-extinction.
In Vitro Revival: Primordial Germ Cell Isolation, Culturing, and CRISPR/Cas9 Genome Editing
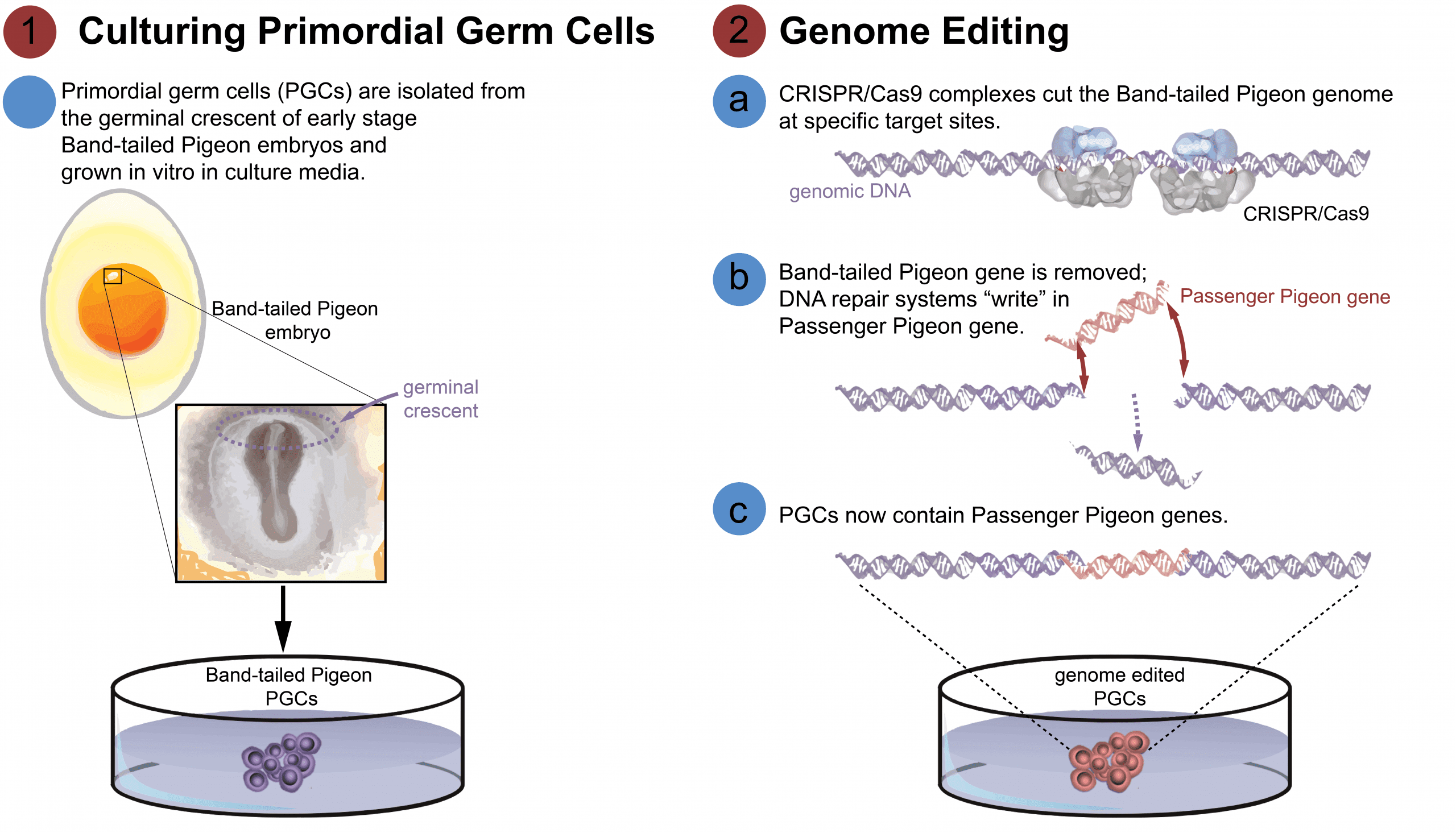
To produce genome-edited birds the most efficient method begins with editing genomes in cell cultures. For birds, the only cells that can be used are Primordial Germ Cells (PGCs) – a kind of embryonic stem cell that becomes sperm and eggs in adult birds.
In a developing embryo, PGCs can be isolated from the germinal crescent, a region in the early-stage embryo where those particular cells form, or from the gonads at a later stage in embryonic development. By studying Rock Pigeon embryonic development, our partner Crystal Bioscience has developed the protocols for isolating PGCs.
Once isolated, these cells can be grown in a liquid medium of nutrients called the culture. Cultured cells will live and grow in the lab, allowing us to make many gene changes without needing to hatch birds every time we make edits to the genome. The right culture conditions for different bird species requires experimentation to develop the correct formula. Developing PGC cultures for pigeons is essential to accomplishing de-extinction.
Once PGCs are in culture, we can use CRISPR/Cas9 technology to edit the genome. The CRISPR/Cas9 system is a “cut and paste” biotechnology – the CRISPR portion works to direct the Cas9 enzyme to a specific site in the genome, where the tool binds and cuts. Once cut, the cell’s own DNA repair mechanisms set to work to mend the break in the DNA molecule – this is an opportunity to introduce alternate DNA codes at the site of the cut.
[A] We will design CRISPR sequences to target specific regions of the genome containing the genes responsible for differences between Band-tailed Pigeons and Passenger Pigeons. Once our custom CRISPR/Cas9 complexes are injected inside a cell, they will bind to the Band-tailed Pigeon genome at those target sites and cut the DNA.
[B] The synthesized Passenger Pigeon DNA from Phase 1.3 is introduced to the cell with the CRISPR/Cas9. After the DNA is cut, the synthesized DNA is integrated into the genome by the cell’s own DNA repair mechanism – homologous recombination. The ends of the synthesized Passenger Pigeon DNA code will match the DNA at the cut sites, allowing the cell to piece together the two strands of codes by binding the matching ends.
[C] Now that the Band-tailed Pigeon DNA has been removed and overwritten with Passenger Pigeon DNA, the process of “allele replacement” is complete. This means the cultured PGCs are now slightly Passenger Pigeon. By repeating this process, we will eventually develop PGCs that contain newly created Passenger Pigeon genomes resembling a sort of hybrid DNA code between modern Band-tailed Pigeons and extinct Passenger Pigeons.
The Goal
The goal is a hybrid genome that produces a bird that looks and behaves like an extinct Passenger Pigeon and that the genetic legacy of the extinct species.
Phase 2.3 – In Vivo Revival: Germ-line Chimera Generation
[A] In order to turn the engineered PGCs into a living bird, the cells will go through a process called germ-line transfer. The PGCs will be injected into the bloodstream of a developing Rock Pigeon, a species adapted for domestic breeding purposes. The cells will migrate and colonize the reproductive organs, or gonads (ovaries or testes), of the embryo. This process is routinely used to generate genome-edited chickens from cultured PGCs.
[B] The embryo develops into an interspecies germ-line chimera, a bird with the body of a Rock Pigeon and the reproductive cells of genome-edited Band-tailed Pigeons, which are our the new Passenger Pigeon cells. A germ-line chimera is not a hybrid bird because every tissue of the bird grew from the host embryo; the DNA of the host and the germ-cells do not intermix. The chimera’s gonads will still contain Rock Pigeon germ-cells as well.
[C] When a male germ-line chimera is mated to a female germ-line chimera they will have three types of offspring: pure Rock Pigeons, sterile Rock Pigeon/Passenger Pigeon hybrids, and when an engineered sperm meets an engineered egg cell, fully formed de-extinct Passenger Pigeon. Genome screening of early developing embryos will allow our breeders to keep only the pure Passenger Pigeon eggs carrying a fully edited set of alleles from each parent. The screening process will allow us to focus all resources on the first generation of revive Passenger Pigeons; these chicks will form the first true generation of de-extinct Passenger Pigeons.
Phase 3.1 – Ex Situ Restoration: Ex Situ Captive Breeding
[A] Generating large numbers of Passenger Pigeons that breed and behave naturally will require several different parent flocks of birds and many generations of breeding. The germ-line chimeras can be continually bred with controlled lighting to produce large numbers of offspring. As many as 50 offspring per year can be fledged from a single pair of birds. A flock of five breeding pairs, each carrying slightly different genetic lines of Passenger Pigeon, can produce nearly 200 offspring per year. Pigeons can live for over ten years and start breeding in their first year. To ensure health and welfare for the birds, pairs will be taken out of cycle periodically. By rotating different sets of pairs in and out of cycle, a flock of six breeding pairs (5 pairs breeding at a time) could produce almost 2,000 offspring in their lifetime. The pairs will be mixed and matched in order to generate genetic diversity.
[B] The eggs from germ-line chimeras will be transferred to surrogate parents for incubation and parenting. The goal is to produce a community of surrogate parents that breed in similar societies to Passenger Pigeons, so that our new Passenger Pigeons develop with the proper behavioral culture. Band-tailed pigeons nest in trees like Passenger Pigeons did, but do not nest in tight communities. Rock pigeons will nest in dense communities, but not on tree branches. Rock pigeons may be trained and raised to use nest platforms on tree branches, or Band-tailed Pigeons may be able to be conditioned to tolerate close proximity. Likely, a combination of surrogate parents will be necessary to foster the first generation of Passenger Pigeons. These captive-bred birds will be housed in environments of natural trees and forest plant species, preventing them from becoming domesticated. Their food will not be supplied in dishes, but strewn about through undergrowth and foliage, stimulating natural foraging behavior.
[C] Once sexually mature, the first generation of new Passenger Pigeons will breed and raise their own offspring in natural cycles, without major intrusions from human caretakers. We want to ensure that these captive-bred birds can thrive in the wild.
The Goal
Eventually, there will be no need for the chimeras and surrogate parents; once the captive population reaches a size that allows individuals to be removed for wild release without endangering the continuance of the flock, we will have reached our goal of a viable breeding population.
Phase 3.2 – In Situ Restoration: In Situ Wild Release
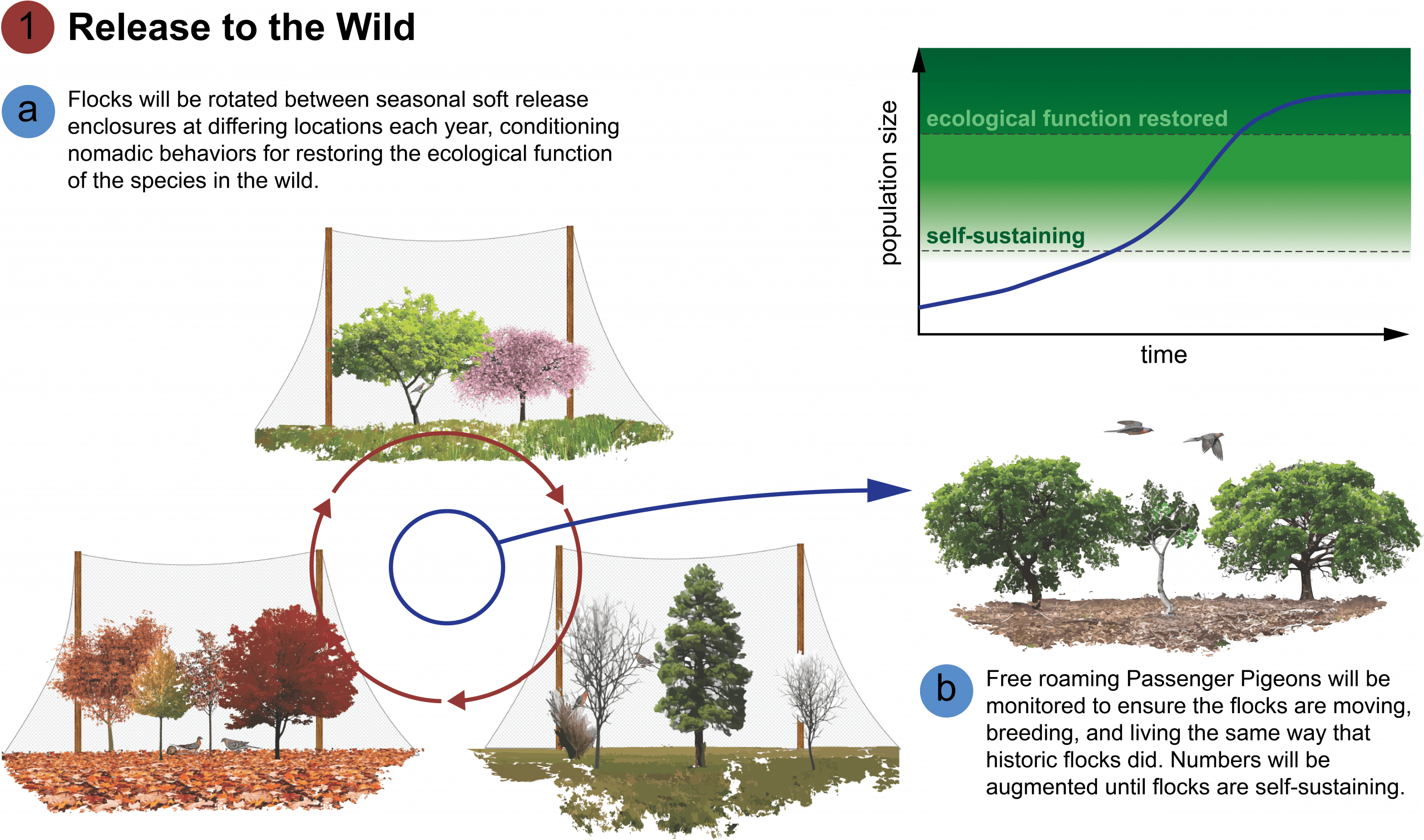
[A] The first step in wild release will be a “soft release.” Large flight aviaries encompassing entire trees set within forest locations will be constructed at two sites: one within the former breeding range (spring locations) and one within roosting/foraging ranges (autumn and winter) of the Passenger Pigeon. These structures will allow flock managers to monitor how the birds cope with natural weather conditions. The enclosures will be designed so that other species (including squirrels, rabbits, and deer) can be introduced and their interactions with the Passenger Pigeon flock can be observed.
Because Passenger Pigeons were nomadic, it is important to prevent the birds from acclimating to specific sites, the birds will be rotated randomly between multiple spring, summer, and winter sites. Moving the birds will be one of the project’s biggest challenges. Cranes and geese raised in captivity have been taught flight movements by airplanes. It may be possible to fly Passenger Pigeons using specialized drones, but a more natural solution may be achievable using homing pigeons as surrogate flocks to lead Passenger Pigeons from one site to another. This strategy poses a big risk: the birds will be completely free temporarily. The birds may scatter and not follow the surrogate flock. All birds will be implanted with micro-GPS trackers to trace their movements. In this way, we can locate and retrieve birds that wander, and more importantly, we can observe if the birds are forming the tight social bonds for flocking. In time, it may be possible to stop using the homing pigeons to lead new flocks of Passenger Pigeons.
[B] Once it appears that the pigeons that were bred and raised in soft release rotation are adapted to natural conditions and flock in a similar manner as Passenger Pigeons did historically, we can begin releasing test flocks to the wild. The first birds free in the wild will still be monitored by GPS for study. Until the flock is self-sustaining, this population will likely be designated as an experimental population for research.
The Goal
Our goal is to release the first test flocks between 2030 and 2040, eventually reaching a target of self-sustaining population growth with 10,000 birds in the wild. This goal is technologically feasible if adequate funding is maintained. The wild population will be stocked from soft release sites until it appears to be self-sustaining. This may be possible in less than twenty years.