COVID-19 Vaccines and the Harvesting, Bleeding, and Killing of Horseshoe Crabs
This article was published on July 01, 2020. Access the original PDF, here.
Wilson Sonsini attorneys Vern Norviel and Charles Andres recently sat down with Ryan Phelan of Revive & Restore (R&R). R&R is using 21st-century biotechnology (including genomics, high throughput sequencing, and synthetic biology) to address urgent conservation challenges.
One issue that is particularly pressing is the harvesting and bleeding of horseshoe crabs, a threatened keystone species, and the source of Limulus amebocyte lysate (LAL). LAL, obtained from the blood of horseshoe crabs, is employed in pharmaceutical manufacturing to test human and animal vaccines and drugs for the presence of bacterial endotoxins.

Inside a Charles River Laboratory bleeding facility. (Timothy Fadek)
In essence, the crabs are harvested in the hundreds of thousands from the Atlantic coast, and then spend 24 to 48 hours being transported by boat to factories, where many of the crabs die in transit. The crabs that survive are impaled with a needle and bled in factory-like settings and drained of about one-third of their blood, as shown in the photo above. After being industrially bled, the crabs are returned to the ocean, where many of them die and others fail to spawn. Because they are technically returned to the ocean, and because LAL is deemed a medical necessity, it is essentially a nonregulated industry.
The race to develop COVID-19 vaccines—and the anticipated production of billions of COVID-19 vaccine doses—highlights the need for making available to pharmaceutical companies a safe and scientifically proven LAL alternative with minimal regulatory hurdles.
Luckily, an alternative to LAL exists in the form of recombinant Factor C (rFC) protein, which can be readily made in more than sufficient quantities to meet present and future pharmaceutical needs. A number of peer-reviewed scientific publications attest to the fact that rFC is a safe, effective, and viable alternative to LAL. For instance, Europe and China have adopted rFC as an LAL alternative.
Major pharmaceutical companies have embraced the so-called “three R Framework”:
- Replace the use of animals with alternative techniques, or avoid the use of animals altogether;
- Reduce the number of animals used to a minimum, to obtain
information from fewer animals or more information from the same number of animals; - Refine the way experiments are carried out, to make sure animals suffer as little as possible. This includes better housing and improvements to procedures that minimize pain and suffering and/or improve animal welfare.
And rFC fits into the three R Framework. In addition, rFC de-risks supply chains that otherwise rely on harvesting an endangered wild animal as their foundation.
Since January 1, 2020 pharmaceutical companies and environmental organizations were expecting the United States Pharmacopeia (USP) to name rFC as an LAL alternative, thereby making it easier for pharmaceutical manufacturers to employ rFC into their manufacturing processes. But on May 29, the USP did just the opposite, reversing an earlier decision to publish the equivalency endorsement. Instead, the USP said they will develop an entirely separate chapter in their guidelines—a process that is likely to take years. R&R seeks to have the USP change course and name rFC as an LAL alternative. With this as background, we introduce Ryan Phelan.

Ryan Phelan, Executive Director and Co-founder of Revive & Restore
Ryan, please introduce yourself to our readers.
Hi, readers. I am a serial entrepreneur with two successful liquidity exits, including DNA Direct. After the second exit, I decided to take a different course: using biotechnology to tackle unmet conservation challenges. To do this, I co-founded R&R, a 501(c)(3) foundation, a little over seven years ago. In essence, I wanted to take the 21st-century scientific toolbox being developed for human medicine and apply it to pressing environmental problems.
Why name the foundation Revive & Restore?
Great question! We wanted to use state-of-the-art biotechnology to revive endangered species and restore their ecosystems. Hence, Revive & Restore.
Can you provide some examples of reviving and restoring?
Sure. One example is our Black-footed Ferret (BFF) project, which we are conducting in conjunction with the United States Fish & Wildlife Service’s (USFWS’s) National Black-footed Ferret Conservation Center. BFFs are endangered and
are indigenous to North American prairies. The USFWS has been doing a fantastic job breeding BFFs and reintroducing
them into the wild. But the BFFs suffer from two disadvantages: low genetic diversity and complete susceptibility to Sylvatic plague. We are studying two approaches to address these problems.
First, we are preparing, in conjunction with partners, to develop a permanent “vaccine” to Sylvatic plague. In essence, we are looking to convert an effective vaccine for plague in ferrets into a permanent inheritable trait. If successful, the genetically vaccinated BFFs would be born with resistance to plague, which today can only be accomplished by capturing the animals and treating them with a traditional vaccine. Although this current approach protects the BFFs from plague, it keeps them reliant on the continual efforts of humans for survival in the wild.
We have multiple projects to develop the necessary technology for inheritable vaccines for BFFs. First, we are developing methods for introducing new genes into the BFF genome using advanced reproductive technologies, like cloning. In parallel, we are using lab mice to test the hypothesis that a gene for an antibody that attacks Sylvatic plague can lead to heritable immunity over many generations.

A BFF at the National Black-footed Ferret Conservation Center in Colorado.
Second, we are looking to increase the genetic diversity of BFFs. Revive & Restore partnered with San Diego Zoo Global to study the genomes of four unique BFF specimens with the goal of understanding the extent of the genetic diversity problem and whether existing genetic resources could restore diversity into the BFF population. Those genomes were from a living ferret representative of the current population and two cryopreserved cell lines of ferrets originally captured from the wild in the 1980s that were not part of the captive breeding population.
The study found that historic genetic diversity could increase diversity in the living population if historic samples were bred back into the population. Cloning could be used to reproduce the historic ferrets and introduce new founding genetics into the population—a first for an endangered species. Please go here for a list of our current projects.
What is the mission of Revive & Restore?
We’re the leading wildlife conservation organization bringing biotechnologies to conservation. Genetics and genomics can help us build a “Genetic Rescue Toolkit” that helps enhance genetic diversity, build disease resistance, facilitate adaptation to climate change, and more. Yet for most conservationists, these tools are still not readily available. Below is a graphic that captures our mission:
What are the organizational components of Revive & Restore?
One important component is our Catalyst Science Fund. We created this fund to lower the barriers of entry and to increase the use of biotechnology by conservationists through the development of the Genetic Rescue Toolkit. In the space of about two years, we have raised $6M, which we are using to fund projects aimed at demonstrating the application of biotechnologies to problems that have traditionally been the hardest for conservation to solve. For example, Revive & Restore has reserved $1.2M in funding for a new program we just launched called Wild Genomes, which will award research grants over the next two years to scientists addressing a clear conservation need. This program will accelerate the genomic sequencing and biobanking of threatened species and put the fundamental tools of genetic rescue directly into the hands of those who manage wildlife.
Why is this important?
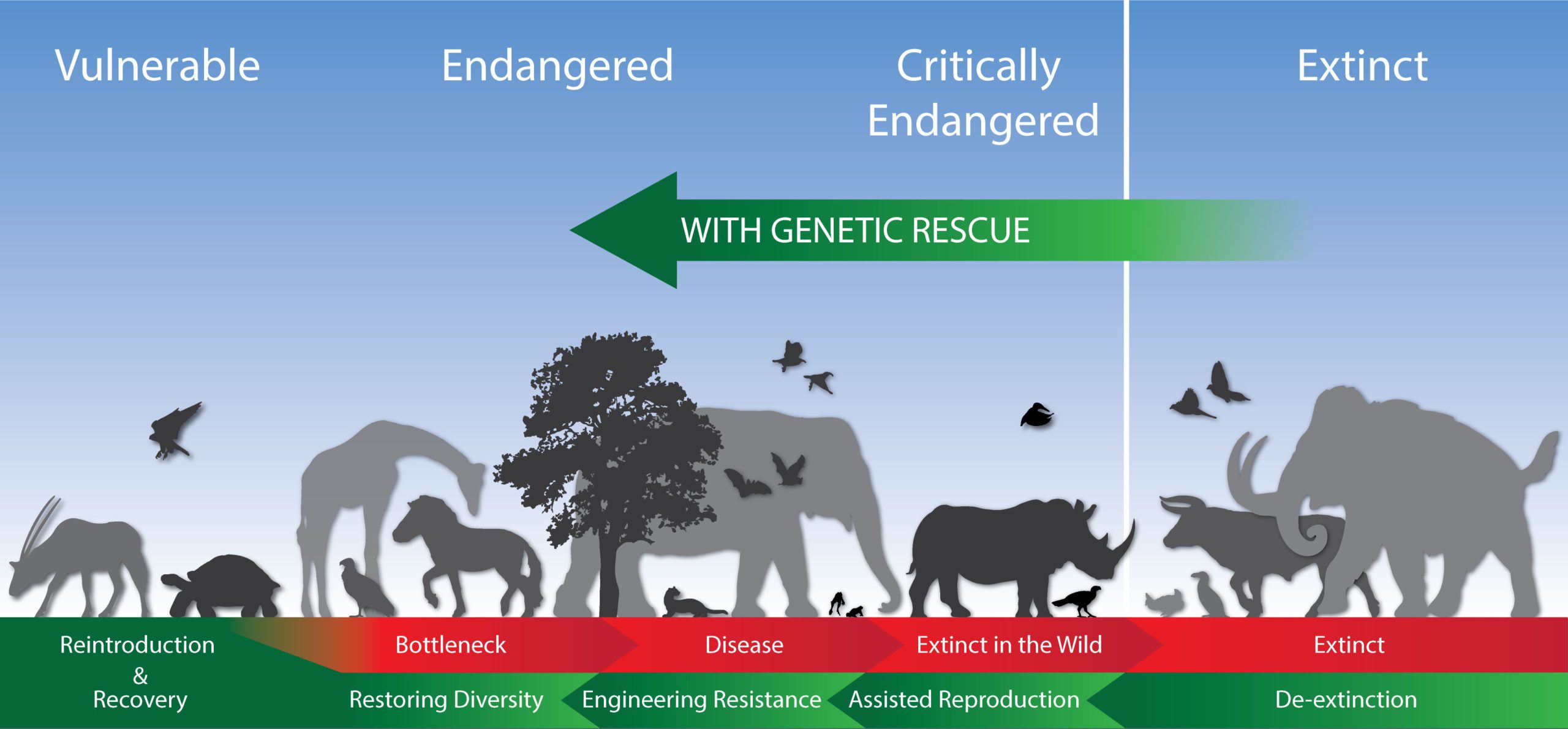
We are in a present-day extinction crisis. We know that we have changed the environment significantly over the last 200 years—and the question is: what do we want to do in the next 200 years? Without addressing biodiversity issues, we will see increasing pathogens (e.g., Ebola, COVID-19) and more challenges to human health. Put differently, humans are part of nature, and humans cannot escape the effects of the degradation of the natural environment. The below graphic shows where we were, where we are, and where we want to move towards: a more bioabundant future.

Let’s talk more about bleeding horseshoe crabs. Why do you think the USP changed course?
The motives behind the USP’s change in course remain unclear—and as we say in the horseshoe crab world, it smells fishy. What incentive could the USP have to further delay the widespread use of rFC? Who is exerting pressure on the USP? There are several companies that have a vested interest in the bleeding of the horseshoe crabs.
At the same time that the USP changed its course, it also announced that in light of the global pandemic, COVID-19 vaccine manufacturers can utilize rFC for endotoxin testing and that the USP would provide assistance to do so as part of their Trust Accelerated program.
The USP’s announcement on May 29 appears contradictory: While the USP tells manufacturers that they must provide real-world evidence of rFC equivalence, the USP is also aiding its use for COVID-19 vaccine development.
This announcement seems purpose-built to absolve the USP from any liability. As companies ramp up to produce at least 14 billion coronavirus vaccines, there will undoubtedly be an increasing demand for endotoxin testing. By encouraging vaccine manufacturers to use rFC, the USP cannot be blamed if the supply chain of horseshoe crab blood becomes untenable. But, by continuing to place the burden of proof on pharmaceutical companies, the USP punts regulation to the FDA approval process while seemingly keeping the USP in good standing with the horseshoe crab bleeders.
But a few pharmaceutical companies, like Eli Lilly, are already using rFC. Plus, the European Pharmacopeia this July has published the guidance that was expected from the USP.
What does R&R want from the USP?
We want the USP to do what they said they were going to do: 1) honor the existing science and data; and 2) do what pharmaceutical companies are urging them to do. We want the USP to reverse their decision on moving ahead with a separate chapter and demanding further efficacy studies. rFC does not need to be in its own stand-alone chapter in the pharmacopeia. Why? Several reasons, including:
- We know there have been over 200 pharmaceutical products studied using rFC, providing significant data with vaccines, raw materials, and large- and small-molecule drug products. Our own 2018 review article published in PLOS Biology of 10 rFC efficacy studies demonstrated that commercially available rFC tests detect endotoxins with results equivalent to or better than LAL, regardless of which company manufactured it. The breadth of these studies also showed strong efficacy across a range of uses and demonstrated high sensitivity, strong reliability, and other positive considerations in the clinical use of rFC.
- We see no evidence-based reason not to declare rFC equivalent to LAL. rFC is made from the horseshoe crab gene that codes for the active component in LAL. But, because LAL includes other factors, it offers less consistent results! Indeed, because LAL suffers from beta-glucan interference, rFC could be deemed superior.
- Removing all unnecessary barriers that prevent the broader use of rFC would give pharmaceutical companies another valuable tool at their disposal to quickly bring forward safe and effective vaccines and therapeutics.
- Both the European Pharmacopoeia and the Chinese Pharmacopoeia have recently recognized rFC. The U.S. is sorely lagging behind, and more importantly, delaying and jeopardizing global harmonization across the pharmacopeias.
- Given such equivalency, the USP should also consider the larger context of animal welfare and environmental conservation.
- Hundreds of thousands of crabs are subjected to capture, time out of water in harsh conditions, and bleeding, and then returned to a location very different from where they were originally caught.
- The horseshoe crab plays a key role in the ecosystem. Thousands of migratory birds critically depend on the spawning of the horseshoe crabs as they refuel on their eggs during the spring migration.
- The potential for a crash in horseshoe crab numbers, as happened 30 years ago, could imperil endotoxin testing and hence the supply chain for critical vaccines and therapeutics.
- Because LAL is an essential component of vaccine production, by destroying the only source of this natural product, these companies are potentially creating a downstream national security issue.
What does R&R want from pharmaceutical companies?
We would like to see more pharmaceutical companies adopt rFC. Just testing the water used in manufacturing with rFC pharma could reduce its reliance on LAL by 90 percent. Doing so is the right thing for the environment and for public health, and would reduce supply chain risk for a component that is essential for vaccine manufacture because current processes rely on non-sustainable harvesting of a wild animal. We also would ask pharmaceutical companies to write the USP and demand that the USP remove all barriers to adoption of rFC.
How can people get involved with R&R?
There are many ways to help. Perhaps the most direct way to make a difference is to contribute to our Catalyst Science Fund. Every contribution helps fund the science needed to secure a more biodiverse future. We are building a new community of biotechnologists focused on creating solutions, not just measuring the decline of the natural world. It’s an exciting and optimistic way to get involved in conservation, and there is such a wide range of species that can be helped by this approach. Corporate contributions helped jumpstart the fund (Promega Corporation donated the first $3M). We’ve been able to match that contribution so far, and now intend to grow the fund to $10M.
Is there anything else you would like readers to know?
In light of COVID-19, we all realize how human health and the environment are intimately intertwined, and the importance of using cutting-edge science to solve urgent problems. R&R is one small part of this and relies on the involvement of people from all walks of life. Our effort to grow the Catalyst Science Fund is instrumental to that success. It is often difficult for researchers to find funding to utilize the tools of biotechnology, like genomic sequencing, in their conservation work because it is so experimental. Catalyst Science Fund grants enable these researchers to generate the first proofs of concept for these new approaches, which can lead to increased interest by the larger funding community.
How can readers get in touch with you?
I can be reached by email at [email protected].
This interview was originally published in Wilson Sonsini’s Summer 2020 Life Sciences Report.