NEW TOOLS
- What technology advances makes genomic treatment possible today?
- What is the new genetic technology called “gene drive”?
- Why are gene drives considered controversial?
- What situations might warrant the consideration of genetic engineering as a solution?
- Who decides when new technologies should be used for conservation?
- Are there guidelines for the use of genomic technologies in conservation?
- How can communities weigh the pros and cons of these new tools?
- Will these new genomic tools be a “magic bullet”?
INVASIVE RODENTS
AVIAN MALARIA
- Are there any native mosquitoes to Hawaii?
- How does avian malaria affect the native birds?
- Might Hawaiian forest birds develop resistance to avian malaria if we let evolution take its natural course?
- Does climate change complicate the survival of Hawaiian native birds?
- Has a genetically engineered mosquito ever been released in the wild?
- Can you engineer the mosquito to stop spreading disease without getting rid of them?
- Which approach requires on-going release? Which is most sustainable?
PLANT FUNGAL DISEASES
What is the goal of the IUCN workshops on “genetic rescue”?
The goal of these workshops is to provide a forum to examine the potential for genomic technologies to solve important conservation problems that have proved difficult to solve with current methods.
How can genetics be used for conservation?
Genomic analysis can reveal not only taxonomic details but also the long-term history of a species and current vulnerabilities, such as inbreeding. Possibilities for genomic treatment include re-introducing genetic variability, increasing a species’ resistance to exotic diseases, and precisely targeted removal of harmful invasive species and of disease vectors.
How big of a problem are exotic wildlife diseases?
Non–native wildlife disease is one of the most significant threats to species conservation worldwide. Notable examples include Hawaiian birds with avian malaria (alien vector: mosquitoes), amphibians with Chytrid fungus, and bats with white-nose syndrome.
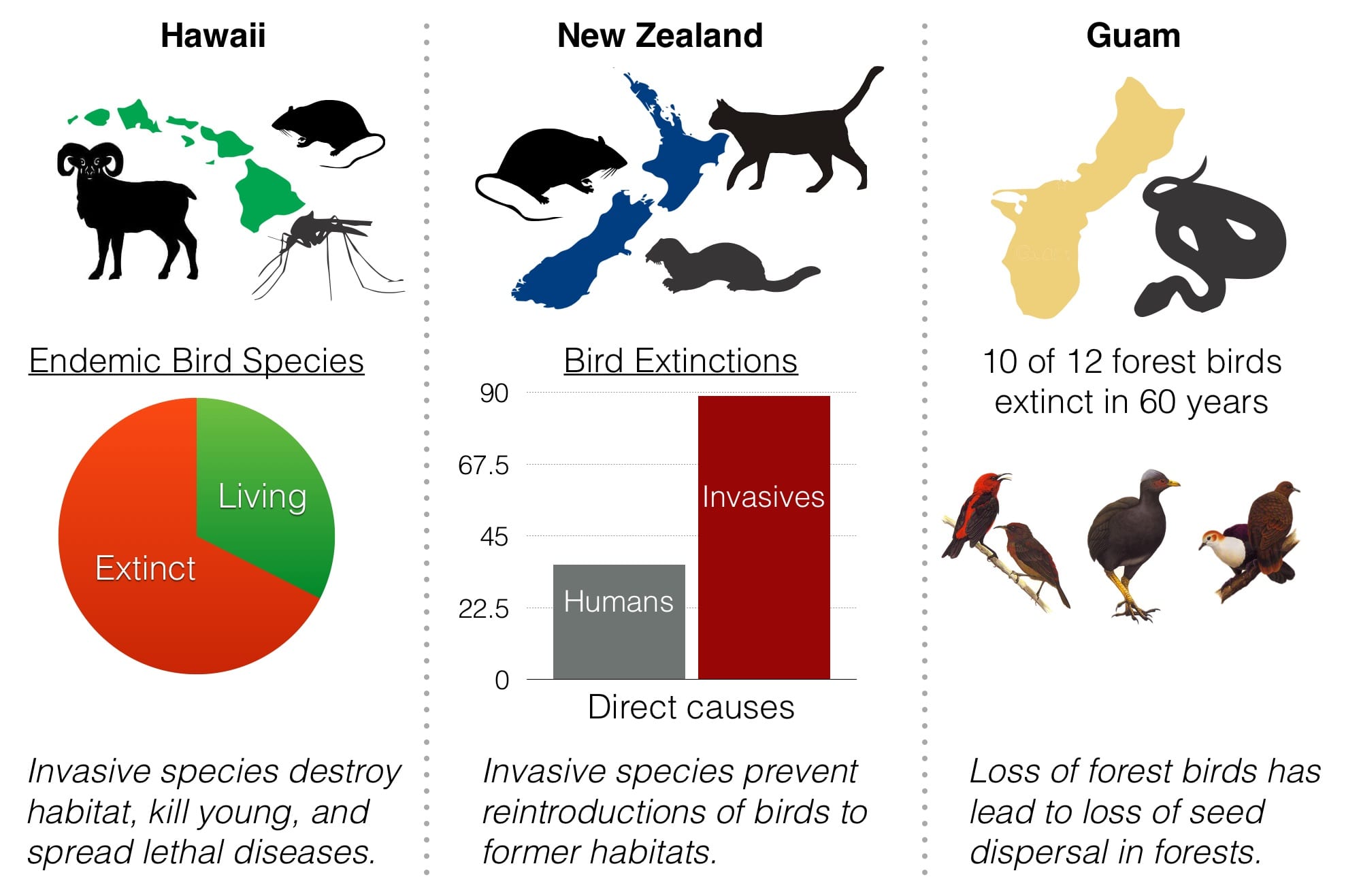
How big of a problem are alien invasive species?
Invasive alien species have a substantial and growing impact globally, economically, culturally, and biologically. Invasive species are responsible for over 50 and 70 percent of recorded vertebrate and plant extinctions, respectively. The overwhelming majority of these extinctions have occurred on islands. Invasive species cost the U.S. $1.2+ billion every year and threaten ecosystems around the globe.
NEW TOOLS
What technology advances make genomic treatment possible today?
Extremely precise genome-editing techniques such as CRISPR/Cas9 now make it possible to adjust the genes of endangered species toward better resistance to exotic diseases, or to modify the genes of invasive species to reduce their ability to reproduce. CRISPR/Cas9 is a genome editing technique adapted from a naturally occurring bacteria immune response. The acronym CRISPR stands for “clustered regularly interspaced short palindromic repeats”.
What situations might warrant the consideration of genetic engineering as a solution?
When a species is on the brink of extinction, and no other methods have proved successful, land stewards consider captive breeding or cryopreservation as the only hope for reintroducing the species at a later time, when heir habitats are safe. When invasive species take such a toll on the native species that whole ecosystems and their future is at risk, land stewards bring out some of the most aggressive and labor intensive options including pesticides, rodenticides, trapping, hunting, often with significant implications for human, animal, and ecosystem health. These are the types of situations in which genetic engineering might be considered, after careful deliberation of the risks and benefits of each approach.
Who decides when new technologies should be used for conservation?
Depending on the technology in question, these genomic tools have a local, regional, and global impact. Public understanding of the pros and cons of using the technology for conservation, and the consequences of not using it, is essential. The public must be engaged in the approval process starting now, before the regulatory process begins.
How individual countries will handle regulation is difficult to predict. In the United States, regulatory agencies, including the Environmental Protection Agency, the Food and Drug Administration, and the U.S. Department of Agriculture will likely all need to approve any new technology of this type being deployed in the environment.
Are there guidelines for the use of genomic technologies in conservation?
Guidelines are being broadly discussed in various countries and by various global bodies. Discussion of the application of these tools in human medicine and agriculture are much more advanced and should help inform the development of conservation guidelines.
Excellent resources that conservation can draw upon include:
- Gene Drives on the Horizon – National Academy of Sciences
- The Guidance Framework for Testing Genetically Modified Mosquitoes
How can communities weigh the pros and cons of these new tools?
When considering genome engineering it is important to understand which method is being contemplated, and under what circumstances, to make the most informed opinion on the pros and cons. It is important to not only think about the impact of the use of these new tools but also what will happen if a genomic solution is not applied.
Will these new genomic tools be a “magic bullet”?
Genomic tools may well turn out to be cheaper and safer than existing approaches, but in any case, they need to be integrated into current protocols and approaches for conservation practice. Risks and benefits need to be thoughtfully and thoroughly considered and weighed by all stakeholders: the general public, local landowners and resource users, local government and agencies and federal government and agencies.
AVIAN MALARIA
Are there any native mosquitoes to Hawaii?
No. There are over 3,800 species of mosquitoes in the world, but before Europeans arrived, Hawaii had none. The first mosquito species was introduced there in 1826. There are now six species of mosquitoes in Hawaii and all are invasive aliens.
Two of these mosquitoes (Aedes aegypti and Aedes albopictus) can carry human diseases including Dengue, Yellow Fever, Chickungunya, West Nile Virus, and Zika. Only one species, Culex quinquefasciatus, carries avian malaria and avian pox, diseases so deadly to the native birds, and so virulent, that they have contributed to extinction of entire species of birds.
How does avian malaria affect the native birds?
Culex quinquefasciatus carries a pathogen called Plasmodium relictum, which reproduces in the birds red blood cells, eventually causing the cells to rupture. Birds die painfully of low blood-oxygen levels and enlargement of the liver and spleen. Because these native Hawaiian birds evolved without needing resistance to avian malaria, they are very susceptible to this disease. And, as the changing climate forces temperatures higher, the range of these mosquitoes increases while the higher-altitude (and therefore cooler) refuges of native birds plummet.
All efforts to limit the mosquitoes with insecticides and other methods have been ineffective or ultimately failed. And the calamitous loss of native birds to avian malaria is a key factor in the Hawaii’s high extinction rate.

Might Hawaiian forest birds develop resistance to avian malaria if we let evolution take its natural course?
Unfortunately, evolution takes time and time is scarce. There are now only 21 species of native forest birds remaining out of an original total of 46 or more. Only one species, the native Amahiki (Chlorodrepanis virens), which occurs in low elevation areas on Hawaii Island and Oahu, shows some indication of developing resistance to avian malaria. However, other bird species are running out of disease-free habitat and the population numbers that would allow the evolution of resistance.
Does climate change complicate the survival of Hawaiian native birds?
Today, most of the remaining native forest birds are endangered, and they survive only at elevations where colder-temperatures limit the spread of mosquitoes (4,500 feet or higher). Climate change models anticipate that the high-altitude safe haven for the Akiapōlāʻau (Hemignathus wilsoni) on Hawaii Island, Puaiohi (Myadestes palmeri) and Akeke’e (Loxops caeruleirostris) and the Akikiki (Oreomystis bairdi) on Kauai, and Kiwikiu (Pseudonestor xanthophrys) and ‘Akohekohe (Palmeria dolei) on Maui will disappear. It is expected ranges will decrease by between 50 and 90 percent by 2100.[1]
Has a genetically engineered mosquito ever been released in the wild?
Aedes aegypti is the mosquito that carries dengue and several other human diseases, including Zika. The biotechnology company Oxitec has developed a genetically modified Aedes aegypti mosquito to suppress the population, without using gene drive. Through genetic engineering, Oxitec has induced sterility in male mosquitoes, so when they are released in the wild, the resulting offspring will inherit an engineered gene that prevents offspring from developing into functional adults.
Oxitec has used this method in field trials in the Cayman Islands, Brazil and Panama on Aedes aegypti. In each case, more than 90% suppression of the target population of mosquitoes was observed.[2] By comparison, the Florida Keys Mosquito Control District reports that intensive application of the most current pesticide achieves an estimated 50% reduction in the normal Aedes aegypti population.[3]
Can you engineer the mosquito to stop spreading disease without getting rid of them?
Yes. “Population replacement” is a method designed to change a species to a less harmful form without killing them. Making the “replaced mosquitoes” less able to transmit a mosquito-borne pathogen is one example. That being said, the mosquitoes will still bite and could be a vector of other diseases. “Population suppression” methods, such as the use of sterile males, aim to reduce the numerical size of the pest population, possibly to zero.
Which approach requires on-going release? Which is most sustainable?
Another distinction made is the degree to which any engineered mosquitoes exist after release. Sterile males will disappear from the environment rapidly if releases cease. This is reassuring in terms of controllability and reversibility, but at the same, requires frequent release of additional modified males during the program to ensure success. A self-sustaining method would require the use of a gene drive designed to persist in the environment and spread within the target population, but with some risk of spreading into a non-targeted area where that same species may be desired. It is anticipated that within the next few years, several versions of gene drive will be developed that build in even more safeguards to eliminate non-target impact.
PLANT FUNGAL DISEASES
What is Rapid Ohi’a Death (ROD)?
Hawaii is facing an unprecedented spread of the invasive fungal pathogen
(Ceratocystis fimbriata). This new fungal pathogen known as Rapid Ohi’a Death was identified on Hawaii Island in 2014. The fungus attacks and can quickly kill Ohi’a trees (Metrosideros polymorpha). The Ohi’a is a keystone species, endemic to Hawaii and comprises approximately 80% of Hawaii’s native forests.
What current treatment approach to ROD is being deployed today?
Although fungicides are being investigated as a tree protectant, it would only be feasible to treat infected trees at an individual level, not at a landscape or ecosystem scale. Initial attempts at containment are underway to reduce inoculum pressure[4] by interrupting the disease cycle as currently understood. It is believed the fungus spreads through the environment by beetles boring into dead infected trees, generating disease-tainted frass[5] that is then spread via wind. By strategically felling large dead trees on the periphery of the infestation and monitoring beetle activity, managers are trying to reduce the spread of the disease. Hawaii is also attempting to limit the spread of the disease by humans through an active outreach program that warns against moving Ohi’a wood.
Preliminary resistance trials are being conducted on different varieties of Ohi’a trees, and scientists are looking at the genetics of both the pathogen and host for potential long-term strategies.
Has there been any success with an engineered approach to treating a plant pathogen?
Yes. The American chestnut (Castanea dentate) is now functionally extinct due to chestnut blight, caused by an exotic fungus, which chokes the trees to death by killing tissue under the bark and obstructing conduits for water and nutrients. The species has survived because new sprouts still grow vigorously when trees die back or are cut down. But they all eventually succumb to the blight, usually before growing old enough to flower and reproduce.
Since the 1980s, scientists at the State University of New York College of Environmental Science and Forestry (SUNY-ESF) have been developing a blight-resistant chestnut tree by engineering in a single gene from wheat. [This gene (which is unrelated to gluten) doesn’t kill the fungus, but rather breaks down a toxin the fungus uses to kill the tree.] Because the species continues to sprout, there is a rich gene pool with which to launch the potential restoration effort led by SUNY-ESF’s American Chestnut Research and Restoration Project. Hundreds of transgenic trees have been produced that still contain 100% of the American chestnut’s native genome, yet they are resistant to blight. Once federal approval is granted, scientists hope to begin planting these trees in the forest, with the goal of restoring American chestnuts to the ecosystems in which they once thrived.
INVASIVE RODENTS
How have invasive alien rodents contributed to extinctions on islands?
Historically, about 80% of extinctions since 1500 have occurred on island ecosystems that today support approximately 40% of all threatened species. One of the greatest threats includes the presence of damaging invasive alien species, particularly rats (Rattus) and mice (Mus spp.). These rodents have been introduced to nearly 90% of the world’s island archipelagos, with new introductions documented every year.
The removal of introduced invasive rats from island ecosystems is a powerful conservation tool to protect threatened species, and has been adopted worldwide, with about 500 successful eradications reported. With the exception of very small islands, each project follows the same fundamental process of eradication – the delivery of a bait containing a rodenticide into every potential territory, timed when rodents are more likely to consume the bait and/or to minimize risks to non‐target species.
Why is a new technique needed to control island populations of mice and rats?
We are always striving in conservation to utilize the best available science in our conservation management efforts to have the minimalist negative impact on the environment. Available rodenticides are not species-specific, and can represent a toxicological risk for non‐target species and human communities. Ensuring that other species are removed prior to the application/delivery of rodenticide is also costly and labor intensive.
How could genome engineering be used to suppress or eradicate invasive rodents?
One approach under development involves a genetically-induced all-male population that is unable to breed. A gene on the Y chromosome called “Sry” induces testis formation and determines sex in most mammals. If the Sry gene is translocated to another chromosome, all of that mouse’s offspring will be male. When coupled with the use of a gene drive naturally occurring in mice, this technique could ensure that all non-native mice on an infested island can be reached to affect an all-male-population unable to reproduce. This would create one of the most humane rodent eradications possible.
A consortium of researchers from North Carolina State University, Texas A&M, USDA, CSIRO (in Australia), and Island Conservation are in a proof-of-concept phase of developing and testing genetic systems of this sort in the laboratory to precisely eliminate invasive rodents from sensitive ecosystems.
References & Notes
[1] http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0140389
[2] http://www.oxitec.com/oxitec-vector-control-solution-in-brazil-attacking-source-of-zika-virus/
[3] http://keysmosquito.org/question-answers-on-gm-mosquitoes/
[4] Inoculum pressure refers to the amount of viable spores in the environment.
[5] The fine powdery refuse or fragile perforated wood produced by the activity of boring insects.