Revive & Restore has set out to expand conservation practice by demonstrating how new genomic tools can be applied to a variety of serious wildlife problems that have proved unsolvable by traditional means.
Working with dozens of scientists, we are participating in 12 such projects—7 initiated by us. Of the 12 projects, 6 aim to prevent extinction of endangered species (genetic rescue), 5 attempt to reverse extinction in ecologically important species (de-extinction), and 1 hopes to cure a devastating human ailment (Lyme disease) by tweaking its wildlife reservoir.
That may seem like too much for a tiny nonprofit to take on, but in fact it is the most efficient approach because all of the projects inform and enhance each other. The pioneering molecular biologists and conservation biologists keep up with the fast-developing new tools and applications through informal communication, as well as formal collaboration. Our online genetic-rescue email forum has 150 scientists discussing the ongoing news.
How does all this relate to funding? With the relevant technologies and scientists all identified and working, the rate-limiting factor now is money. Every bit that comes in to Revive & Restore goes straight to amazing work.
Heath Hen New Englanders fed on this abundant grouse. The birds were market-hunted until they were all gone on the mainland by the 1880s, and the last one died on Martha’s Vineyard in 1932, nationally lamented.
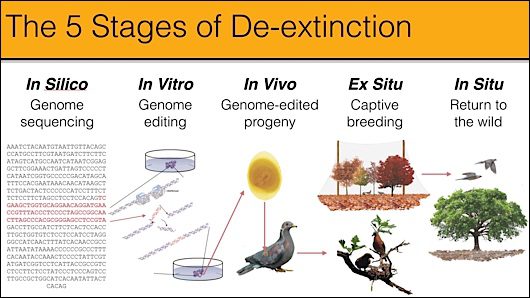
Heath Hens could be the gateway to bringing genetic rescue to all birds. The problem is that you can’t clone a bird the way you can mammals: there’s no uterus in which to implant an embryo with an edited genome. The ingenious workaround is to create “chimeric” parents with edited reproductive cells. Though the stages of that procedure have been demonstrated effectively in chickens, no one has carried out the whole process with a wildlife species.

Heath Hens looked like this greater prairie chicken
In 2014, five funders on Martha’s Vineyard put up $50K to start the project. Working with a handful of volunteer prairie grouse scientists led by Jeff Johnson of the University of North Texas and conservationist Tom Chase on Martha’s Vineyard, Revive & Restore sequenced the genomes of 9 Heath Hen specimens and a variety of prairie grouse to determine the long-questioned evolutionary relationships of the whole family. A start-up company named Dovetail Genomics in Santa Cruz, California, produced the highest quality wild bird genome ever (of the greater prairie chicken) in record time at record low cost. This summer in Martha’s Vineyard, we reported the result, new to science: the Heath Hen was a unique species, uniquely adapted to the New England environment. That makes it a valid candidate for de-extinction.
It is also the ideal candidate for bringing genome editing to birds. Heath Hens are very close to greater prairie chickens genetically. More important, both are fairly close to domestic chickens, which are the most studied of all bird species. There has even been work on chimeric chickens in Scotland, Australia, and California.
Accordingly, Martha’s Vineyard funders are providing $180K (we’re partway there) to cover two years of research on developing chimeric greater prairie chickens by editing the primordial germ cells of their embryos. This would be the breakthrough proof-of-concept and foundational technique for, potentially, all birds. Grouse Park, a breeding facility in Idaho, has built the pens and acquired the greater prairie chickens to provide 40 embryos (eggs) next spring, and we have contracted with Crystal Bioscience in Emeryville, California, to establish the germ-cell resources necessary for genome editing. If it works, then some day soon, greater prairie chickens could be laying fertile Heath Hen eggs, and the first extinct bird would return to the world.
Passenger Pigeon Ben Novak, our staff scientist, has spelled out on our website exactly how the whole Passenger Pigeon revival will play out, from his current genetic and ecological research at UC Santa Cruz, through genome editing and chimeric parents, to eventual restoration of wild flocks to America’s regrown eastern forest to take up their old role of forest regeneration. The first test flocks could be flying by 2032.
A Ben Novak slide
Ben is in the thick of all of our projects, doing the deep contextual research, co-authoring papers, drafting proposals, preparing exquisite slides, and finding scientist allies, often at scientific meetings where he is invited to speak. This year he got married (to Erika Beck) and shortly he will be getting his Masters at UC Santa Cruz. He is pushing the Heath Hen project hard, hoping it will lead the way for his beloved Passenger Pigeons. (There’s a fine chapter about Ben’s work in the new book RESURRECTION SCIENCE: Conservation, De-Extinction and the Precarious Future of Wild Things by M. R. O’Connor.)
Great Auk Called “the penguin of the north,” these flightless pelagic birds thrived throughout the north Atlantic ocean, but their restriction to a few crowded islands for breeding made them vulnerable to exploitation by humans that reached industrial scale. Despite efforts to protect them as early as the 16th century, they were all gone by 1844.

Great Auks by John James Audubon
Last June, a group of 22 specialists gathered in Newcastle, England, to examine the practicalities of reviving and restoring the Great Auk. We were summoned by Viscount Matt Ridley, author of THE RATIONAL OPTIMIST, member of the House of Lords. Tom Gilbert, from the University of Copenhagen, said that his initial sequencing of the Great Auk genome showed that its living relative, the razorbill, is even more closely related than previously thought. The razorbill has similar range and feeding habits and an almost identical appearance, except that it is one-eighth the size and can fly—two traits that may be easy to adjust. The meeting concluded that the project should be pursued, and a follow-up meeting is planned for next year. Meanwhile the group is closely watching our work with the Heath Hen.
Black-footed Ferret The most endangered mammal in North America, Black-footed Ferrets were thought to have gone extinct forty years ago—collateral damage of the war on prairie dogs by high-plains ranchers and farmers. (The Ferrets live solely on prairie dogs.) But then a tiny remnant colony was found, and working with just 7 founding Ferrets, in 1985 a captive breeding program was set up by the US Fish and Wildlife Service. Several thousand Ferrets have been produced, many of them returned to the wild, but the species may be suffering from inbreeding and it is severely threatened by two exotic diseases—slyvatic plague and canine distemper virus. If these problems are not solved, the species could go extinct for good.

Black-footed Ferret and obligate prey
Two years ago, we were invited by US Fish and Wildlife to see if some form of high-tech genetic rescue could be applied. After meeting with the main researchers at Fort Collins, Colorado, we sequenced the genomes of 4 Black-footed Ferrets, 2 from the living population, 2 from cryopreserved tissues at the San Diego Frozen Zoo, that were collected 30 years ago. The genome analysis showed that the living Ferrets may indeed have an inbreeding problem and that the 2 cryopreserved specimens have unique gene alleles not in the descendants of the 7 founders. If they could be cloned back to life, the breeding population would gain the genetic diversity of 2 new founders.
In partnership with San Diego Zoo Global, we are submitting two proposals to US Fish and Wildlife. One spells out how we can clone from the 2 frozen cell lines and put their valuable genes back to work, perhaps via artificial insemination as well as breeding the cloned animals. The second proposal is more radical—to see if we can build resistance to disease into the germline of Black-footed Ferrets and thereby head off their worst threat. As it happens, domestic ferrets are a major lab animal for the study of human diseases, and that will make research on their wild kin much easier.
If these two projects work, they will make conservation history. Neither has been attempted before, and what is learned from the Black-footed Ferrets could be applied to countless others species in need of genetic rescue.
Northern White Rhinoceros They are now down to just 3 living (and nonbreeding) animals left, since the 2 that lived at the San Diego Zoo died this year. But a closely related species, the southern white rhino, is doing pretty well. San Diego Zoo Global has decided to take on the enormous task of using cryopreserved tissue for cross-species cloning to revive the Northern White Rhino, with southern white rhinos as the surrogate parents. (The book RESURRECTION SCIENCE has an illuminating chapter on the rhinos’ predicament.)
The project naturally overlaps with our proposed cloning of Black-footed Ferrets and our attempted de-extinction of another large mammal, the Woolly Mammoth, so Revive & Restore is working closely with San Diego Zoo Global on both the Ferret and Rhino programs.
“New Genomic Solutions for Conservation Problems” Workshop Two breakthroughs transformed genomic technology in the last few years—gene editing with CRISPR Cas9 and the coming of “gene drive” capability which can “drive” even deleterious genes through an entire population. Both have such consequence for conservation that our executive director, Ryan Phelan, determined to put on a workshop to explore how the new tools could be put to practical use. To do it she and Kent Redford raised $160K from individuals and institutions such as The Nature Conservancy, Autodesk, and the National Park Service.
Last April, at a conference resort next to the Golden Gate Bridge, 52 scientists from around the world spent three days doing case studies on: 1) wildlife diseases with vectors such as mosquitoes, 2) wildlife diseases without vectors, such as chytrid fungus in amphibians, 3) destructive island invasives such as rodents and ants. On the last day the three teams took their most promising projects and made business-style pitches to a panel of potential funders. It was electrifying.

The mood at the “New Genomic Solutions” workshop
Not surprisingly, the teamwork that developed at the workshop moved quickly into the real world to carry out some of the projects discussed. In the rest of this report I’ll focus on a few of them.
Asian Elephant Herpes Virus About one quarter of young Asian elephants are dying from an untreatable herpes virus. We’ve learned this when George Church, Ryan, and I paid a visit to the Center for Elephant Conservation in Florida, run by Ringling Brothers.

Not a young Woolly Mammoth—yet
And so we invited to the Genomic Solutions workshop, the leading elephant veterinarian in the US, Dennis Schmitt, and a herpes specialist, Paul Ling. As a result of their case study at the workshop, George Church offered to put his lab at Harvard to work on the barrier to progress—the inability of any lab to culture the virus so that a vaccine can be developed. He would sequence the virus genome fragments, figure out the whole genome, write that, and then culture the resulting virus. That work is nearing completion. Next comes devising the right vaccine or even the right tweak to the elephant genome that would make the animals resistant to the disease. Since we will be asking Asian elephants to help recreate Woolly Mammoths, it seems only right to help out the Asian elephants first.
Woolly Mammoth The most ambitious of all de-extinction projects is the farthest along in terms of actual genome editing. Last year, George Church and his postdocs at Harvard moved 16 genes governing 3 mammoth traits (long hair, subcutaneous fat, and cold-adapted hemoglobin) into living elephant cells. This year, his lab hammered away at making elephant stem cells that will prove expression of the edited traits, and they added genes affecting another mammoth trait—cold insensitivity. His lab is leading the world in bringing new capabilities to everyone’s favorite genome editing tool, CRISPR Cas9. Recently, the lab disabled 62 genes (in a pig genome) all in one go—the largest number of simultaneous gene edits ever accomplished. This bodes well for moving the many genes it will take to convert the Asian elephant genome into an approximate Woolly Mammoth genome.
And now, there is a beautifully written book on the subject, HOW TO CLONE A MAMMOTH: The Science of De-extinction, by Beth Shapiro at UC Santa Cruz, where she does most of our ancient DNA analysis and supervises Ben Novak’s graduate work.
“Sry” Daughterless Mice The greatest conservation successes in recent years have come from cleansing entire islands of the most devastating invasive animals, especially rodents. (See the excellent book RAT ISLAND.) But the vast quantities of rodenticide that have to be used also poison the islands significantly, affecting native wildlife.
What if the rodent population could be used against itself, by genomic means? That was the hope raised at the Genomic Solutions workshop. Of the several island projects that grew out of the workshop, the one that is moving most quickly involves genetically induced “daughterless mice.” A gene on the Y chromosome called “Sry” induces testis formation and determines sex in most mammals. If the Sry gene is translocated to another chromosome, all the offspring will be male, and reproduction of the species will end, with no impact on other species. A consortium of researchers from North Carolina State University, Texas A & M, USDA, CSIRO (in Australia), and Island Conservation are developing genetic systems of this sort to precisely eliminate invasive rodents from sensitive ecosystems. They are also investigating the use of a natural gene drive system in mice to ensure to ensure that all of the non-native mice on an infested island can be reached and made nonreproductive. No mice are killed by this technique; they are just no longer born. (While Revive & Restore is paying close attention to this project, hopefully the first of many, we are not directly involved with its deployment.)
Lyme Disease It is a horrifying human malady now afflicting hundreds of thousands, mainly on the US east coast. People have become afraid to go in the woods or even stroll in tall grass, dreading the infected bite of tiny Lyme-carrying deer ticks.
A leading spokesman and investigator of gene drive is Kevin Esvelt. He and George Church wrote definitive early papers on the subject, and they described the process at the Genomic Solutions workshop. Esvelt, now based at MIT’s Media Lab, wants to see if gene drive can eliminate Lyme disease. He notes that though ticks carry the disease, its real reservoir in the wild is white-footed mice, and their reproductive behavior is more susceptible to gene drive than ticks. If Lyme-resistance can be edited into the mice and spread through the wild population via gene drive without affecting their fitness, the disease could be reduced or elminated with zero ecological impact. That research is under way, along with efforts to make sure the public is included in the whole process. (This project is entirely Kevin’s. We’ll help any way we can.)
Avian Malaria in Hawaii The extinction hotspot in the world is Hawaii, mainly because of the calamitous loss of native birds to avian malaria. It is an alien invasive disease carried by alien invasive mosquitoes. All efforts to limit the mosquitoes with insecticides and other methods have failed.
As with the invasive mice, what if the mosquitoes can be used against the mosquitoes? We don’t even have to wait for gene drive capability to be fully developed. In a presentation titled “Paradise Regained,” the team at the Genomic Solutions workshop proposed deploying a technique used by the British firm Oxitec in Brazil to defeat mosquitoes carrying human dengue fever. Called RIDL (for “Release of Insects with Dominant Lethality”), it involves releasing large numbers of male mosquitoes with their genes adjusted so that when they mate with wild females, the offspring don’t survive. No mosquitoes, no disease. (Revive & Restore plans to participate in this one.)
Cautionary Vigilance Too often, new technologies arouse public worries in a way that paralyzes progress. What we need is a tool that invites the public into the research process–a public forum where debate can play out in an evidence-driven way. Revive & Restore is now prototyping such a tool.

Thanks to support from Jack Newman, cofounder of Amyris, we are building “Cautionary Vigilance” as a website forum designed to dissect the complexity of a controversial innovation into a logical array of specific debates curated to get resolved, over time, by evidence. Preliminary public testing of the first version will begin in early 2016.
Always worth asking: “What would the victory condition of these projects look like?“
How about: 1) Heath Hens back and all birds genetically treatable; 2) Passenger Pigeons back; 3) Great Auks back; 4) Black-footed Ferrets disease-free; 5) Northern White Rhinos back; 6) Genetic-rescue tools in wide and responsible use; 7) Asian elephants liberated from lethal herpes; 8) Woolly Mammoths back; 9) Islands liberated from invasive rodents; 10) Lyme disease rare; 11) Hawaiian birds liberated from malaria; 12) A debate tool in use by the general public to work through controversy about new technologies.
Not immediately. But soon enough if we bear down.
—Stewart Brand